Infrared Spectroscopy
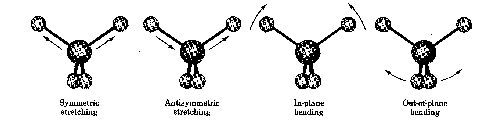
When the molecule is irradiated with electromagnetic radiation, the vibrating bond will absorb energy if the frequencies of the light and the vibration are the same. This absorption phenomena is the principle the IR takes advantage of in the production of spectra that can be easily interpreted by the trained chemist.

The above graph, of an unknown sample, shows specific regions where the vibrations are characterized. The IR is a popular tool used by inorganic, organic, and physical chemists due to the variety of information it conveys in a fast amount of time.

Nicolet iS5 FTIR (2015)

Nicolet 380 FTIR (2006)
Dr. Michael Norton
Dept. of Chemistry
Email: norton@marshall.edu
Phone: 304-696-3489
Dr. Gary Anderson
Dept. of Chemistry
Email: anderson@marshall.edu
Phone: 304-696-6594
Dr. Michael Castellani
Dept. of Chemistry
Email: castella@marshall.edu
Phone: 304-696-6486
Nicolet iS50 FTIR with Smart Diffuse Reflectance (2014)
Dr. Rosalynn Quinones
Dept. of Chemistry
Email: quinonesr@marshall.edu
Phone: 304-696-6731

Matrix Isolation FTIR: Bruker FTIR with pyrolysis unit and cryostat cell (2009)
Dr. Laura McCunn
Dept. of Chemistry
Email: mccunn@marshall.edu
Phone: 304-696-2319

Bruker Optics Vertex 70 FTIR Spectrometer (2008)
Dr. Laura McCunn
Dept. of Chemistry
Email: mccunn@marshall.edu
Phone: 304-696-2319
UV/Vis Spectroscopy
Ultraviolet spectroscopy is applicable solely to conjugated systems. This is because most organic compounds produce no effect whenever the relatively high-energy radiation that constitutes the ultraviolet (200–400 nm) and visible (400–700 nm) portion of the electromagnetic spectrum are presented. Whenever ultraviolet radiation is used, the energy absorbed by a molecule corresponds to the amount necessary to excite electrons from one molecular orbital to another. Typical organic compounds, which mostly contain s bonds, require much higher wavelengths to become excited than what the range of UV will provide. Only those conjugated systems with a number of p bonds are capable of being promoted to higher energy levels by ultraviolet and visible light.
A sample is placed into a cuvette and the spectrum is recorded by irradiating the sample with ultraviolet light of continuously changing wavelength. When the wavelength of light corresponds to the energy level required to excite an electron to a higher energy level, energy is absorbed. This absorption is detected and plotted on the vertical axis as the absorption while the wavelength of the absorbed light is plotted horizontally.

2 Cary 50 Bio UV-VIS Spectrophotometers (2006)
Dr. Michael Norton
Dept. of Chemistry
Email: norton@marshall.edu
Phone: 304-696-3489
Dr. William Price
Dept. of Chemistry
Email: pricew@marshall.edu
Phone: 304-696-3156

GE NanoVue UV/Vis Spectrophotometer (2008)

Shimadzu UV-1800 UV/Vis Spectrophotometer (2009)
Dr. Derrick Kolling
Dept. of Chemistry
Email: kolling@marshall.edu
Phone: 304-696-2307

BioTek Epoch Microplate Spectrophotometer (2009)
Atomic Absorption Spectroscopy

Varian SpectrAA-600 AAS with a GTA-100 Zeeman Graphite Furnace (1994)
Dr. Aley El-Shazly
Dept. of Geology
Email: elshazly@marshall.edu
Phone: 304-696-6756
Atomic Emission Spectroscopy

Varian Liberty 110 ICP-AES (1994)
Dr. Aley El-Shazly
Dept. of Geology
Email: elshazly@marshall.edu
Phone: 304-696-6756
Fluorescence Spectroscopy

SPEX Fluorolog III lifetime fluorometer (1998)
Dr. Robert Morgan
Dept. of Chemistry
Email: morganr@marshall.edu
Phone: 304-696-3159

ISS PC1 Photom Counting Spectrofluorometer (1998)
Dr. Michael Norton
Dept. of Chemistry
Email: norton@marshall.edu
Phone: 304-696-3489

PerkinElmer LS 55 Fluorescence Spectrometer (2009)

Photon Systems Instruments FL 3500 Fluorometer (2009)
Dr. Derrick Kolling
Dept. of Chemistry
Email: kolling@marshall.edu
Phone: 304-696-2307